靶向EphA2:對抗腫瘤耐藥與轉移的新焦點
日期:2025-11-10 17:05:25
腫瘤的耐藥與轉移,是導致癌癥治療失敗與患者死亡的主要原因。面對這一臨床困境,科學家們將目光投向了癌細胞上一個關鍵的“信號樞紐”——EphA2受體。
大量研究表明,EphA2不僅在多種癌癥中高表達,更在驅動耐藥形成、促進侵襲轉移中扮演著核心角色。本文將深入解析EphA2獨特的雙重信號機制,闡述其如何成為腫瘤惡化的“幫兇”,并盤點圍繞它展開的全新靶向治療策略,為您揭示這一充滿潛力的抗癌新焦點。
1. EphA2受體酪氨酸激酶的研究背景
EphA2(Erythropoietin-producing human hepatocellular receptor A2)是Eph受體酪氨酸激酶(RTK)家族的重要成員,該家族是已知最大的RTK亞型,在細胞通訊、發育、形態建成、遷移及黏附中發揮核心作用 [1,2]。EphA2的激活依賴于配體結合,能夠形成不同構象的寡聚體,引發多樣化信號反應 [3],因此在生理與病理狀態下均具有廣泛影響。
大量研究證實,EphA2在多種惡性腫瘤中高表達,并與腫瘤侵襲性、轉移及不良預后密切相關 [4-6]。在三陰性乳腺癌中,EphA2高表達與較差生存率顯著相關,其基因沉默可抑制細胞增殖與侵襲 [4]。類似的促癌效應也出現在結直腸癌 [5]、胃癌 [7]、卵巢癌 [8]、非小細胞肺癌 [9]等。EphA2可促進腫瘤細胞增殖、上皮-間充質轉化(EMT)[10]、血管生成 [7,11]、轉移及耐藥形成 [12,13]。此外,外泌體EphA2能在細胞間轉移耐藥性,進一步加劇腫瘤惡性進程 [12]。
EphA2信號具有典型的“雙重功能”,即配體依賴性(經典)與配體非依賴性(非經典)模式。經典信號多表現為抑癌作用,例如ephrinA1-EphA2結合可通過Ezrin磷酸化調控肌動蛋白骨架,促進細胞極化與黏附 [1,2];而在缺乏配體的腫瘤環境中,EphA2常通過Ser897位點的磷酸化形成非經典信號,呈現促癌特征 [14-16]。例如,前列腺癌中雄激素受體(AR)抑制作用的缺失會導致EphA2過表達,并經非經典信號驅動去勢抵抗 [6,15]。除腫瘤外,EphA2亦參與炎癥與免疫過程,如LPS誘導的肺損傷 [17]及鼻黏膜抗病毒反應 [18,19]。
因此,EphA2因其在多種疾病中的關鍵作用及信號復雜性,成為具有挑戰性的潛在治療靶點 [20,21]。目前針對EphA2的小分子抑制劑、單克隆抗體、ADC藥物及納米遞送系統均在積極研發中 [22,23],如抑制劑ALW-II-41-27能抑制宮頸癌生長并逆轉結直腸癌耐藥 [5,24]。此外,EphA2與PARP或HDAC抑制劑聯合治療在卵巢癌中展現協同效應 [25,26]。因此,深入理解EphA2信號通路對于靶向治療開發與耐藥機制研究具有重要意義。
2. EphA2的分子背景、結構與基本功能
2.1 Eph受體家族與ephrin配體概述
Eph受體酪氨酸激酶(Eph RTK)家族是介導細胞間通訊的重要分子網絡,依據序列同源性與配體特異性分為EphA與EphB兩類。EphA受體主要結合GPI錨定型ephrinA配體,EphB受體則與跨膜型ephrinB結合 [8]。與其他RTK不同,Eph/ephrin信號依賴細胞間直接接觸,其激活需跨膜形成受體-配體復合物 [27]。這種結構特征使EphA2在細胞識別、遷移和形態變化中發揮關鍵作用。例如,EphA2與ephrinA1結合能調節細胞黏附與排斥反應 [1,2],在發育與腫瘤生長中均有重要意義。不同ephrin配體(如EFNA1與EFNA5)在疾病背景中可能具有相反效應 [8],體現了Eph/ephrin信號的高度情境依賴性。
2.2 EphA2結構特點與表達模式
EphA2結構包括胞外配體結合域、跨膜區與胞內酪氨酸激酶域。胞外域負責識別EphrinA配體,胞內域含近膜區、激酶結構域及PDZ結合基序,用以募集下游信號復合物 [3]。其激活常伴隨受體二聚化與自磷酸化。研究表明,膜膽固醇降低可促進EphA2自組裝及Ser897磷酸化,增強其促癌信號 [28]。
EphA2在正常組織中廣泛表達,如血管平滑肌細胞和鼻竇黏膜 [19,29]。但在多種惡性腫瘤中,其高表達是典型特征,與侵襲、轉移及預后不良密切相關 [20,31]。例如,在TNBC中EphA2促進細胞增殖和骨轉移 [32];在胰腺癌中,其過表達增強藥物遞送效率并提高吉西他濱療效 [33]。此外,EphA2的高表達與鼻咽癌放療抵抗 [34]、前列腺癌去勢抵抗 [15]、胃癌B3GNT3上調 [35]及肝癌CD90陽性細胞遷移 [36] 均密切相關。EFNA5的過表達也與卵巢癌患者生存期縮短相關 [8]。因此,EphA2不僅是致癌信號樞紐,也是潛在生物標志物與治療靶點。
2.3 經典與非經典信號傳導模式
EphA2信號具有兩種主要模式:配體依賴性(經典)與配體非依賴性(非經典)通路。
經典信號由ephrinA1結合觸發,通過受體寡聚化和酪氨酸殘基自磷酸化激活下游通路 [37]。例如,EphA2在血管平滑肌細胞中與ephrinA1結合可抑制ERK1/2與AKT活性,從而抑制細胞遷移 [29]。外泌體EphA2在內皮細胞中經EphrinA1依賴通路激活AMPK信號,促進血管生成 [11]。
非經典信號則在缺乏配體時被激活,典型標志為Ser897磷酸化 [14]。例如,在前列腺癌中,EphA2 S897磷酸化促進去勢抵抗與侵襲 [6,15];在血管平滑肌細胞中,該位點磷酸化可增強遷移 [29]。MK2-RSK信號軸被認為是EphA2非經典激活的重要上游調節因子 [14]。此外,EphA2可被MT1-MMP裂解為促癌性EphA2-NF片段,成為潛在腫瘤標志物 [39]。因此,經典信號常表現抑癌效應,而非經典信號多與腫瘤進展相關。
3. EphA2的信號通路與研究機制
EphA2信號網絡復雜,涉及多條細胞內通路的交叉調控,尤其是PI3K/AKT、MAPK/ERK、Rho GTPase及Src家族通路。其激活方式(配體依賴或非依賴)直接影響這些下游信號的方向與強度。
3.1 配體依賴的經典信號通路
配體結合誘導EphA2二聚化與酪氨酸自磷酸化,形成經典前向信號 [3]。EphA2-ephrinA1信號可通過Ezrin-RhoA通路調節細胞骨架與極性 [1];通過Src-FAK-paxillin軸調控細胞黏附與遷移 [27];并在血管平滑肌細胞中抑制ERK/AKT信號,阻止病理性增殖 [29]。此外,EphA2激活后還可誘導配體ephrin-A反向信號,如外泌體EphA2通過EphrinA1激活ERK1/2或AMPK通路,促進腫瘤侵襲與血管生成 [11,12]。這表明經典信號具有雙向性,既維持細胞穩態,也在特定背景下調節腫瘤進展。
3.2 配體非依賴的非經典信號通路
在缺乏配體或特定應激下,EphA2通過Ser897或Tyr772位點磷酸化形成非經典激活模式 [14-16]。Ser897磷酸化由RSK介導,并在前列腺癌、肝癌及血管平滑肌細胞中增強遷移和侵襲 [15,29,36];化療藥物如順鉑亦可通過ERK-RSK-EphA2通路誘導S897磷酸化,導致卵巢癌耐藥 [40]。Y772位點磷酸化則激活Shp2/ERK1/2信號,驅動鼻咽癌細胞增殖 [16]。此外,MT1-MMP裂解、膽固醇降低及SNAI1-PIK3R2相互作用均可促進EphA2非經典信號活化 [10,28,39]。這些機制共同塑造了EphA2在腫瘤中的促遷移、促侵襲及耐藥特性。
3.3 與主要信號通路的交互調控
EphA2與PI3K/AKT通路廣泛交聯,常通過上調AKT信號促進細胞存活與遷移 [7,13,35,41,42]。在胃癌與乳腺癌中,EphA2激活PI3K/AKT通路促進惡性進展 [4,35];而在PTEN缺失背景下,Src激活亦可上調EphA2表達 [45]。
MAPK/ERK通路是另一核心軸,EphA2 S897磷酸化常依賴ERK-RSK信號 [14,46],其抑制可恢復化療敏感性 [40]。
此外,EphA2調節Rho GTPase家族以控制細胞骨架重塑 [1,24];與Src激酶協同調節粘著斑動態 [27,45];并可通過Wnt/β-catenin通路驅動EMT與干性維持 [10,44]。
這些復雜的信號交互構成了EphA2作為“信號樞紐”的生物學基礎,也為靶向治療提供了多層干預節點。
4. EphA2在相關疾病中的作用
EphA2受體酪氨酸激酶作為細胞信號傳導的關鍵分子,在多種病理過程中具有廣泛影響。本章重點概述其在腫瘤發生發展、心血管疾病及炎癥反應中的功能機制,強調EphA2信號通路在疾病演進和治療中的多重角色。
4.1 EphA2在腫瘤發生發展中的作用
EphA2在多種實體瘤中表現出促癌作用,通過調控細胞增殖、遷移、侵襲、EMT轉化、血管生成及耐藥性形成,驅動腫瘤惡化。以下選取幾種研究最深入的癌癥類型進行概述。
4.1.1 前列腺癌與去勢抵抗
在前列腺癌(PCa)中,EphA2高表達與去勢抵抗性進展密切相關 [6]。其S897位點的非經典磷酸化可激活致癌信號,促進細胞遷移、侵襲及去分化 [6,15]。磷酸化蛋白質組學研究揭示,EphA2激活可引發PI3K/AKT/mTOR及ERK/MAPK通路廣泛改變,涉及細胞骨架重塑、運動與連接調節 [38]。盡管EphA2具有促癌與抑癌雙重潛能,但在CRPC背景下,其非經典信號明顯偏向促癌方向。SHB與afadin等銜接蛋白進一步調節EphA2-ERK通路,增強細胞遷移與侵襲能力 [38]。因此,阻斷EphA2非經典信號被認為是延緩PCa進展的潛在策略。
4.1.2 胰腺癌與早期診斷潛力
EphA2在胰腺導管腺癌(PDC)中過表達,是早期診斷與藥物遞送的潛在靶點 [33]。研究顯示,EphA2靶向吉西他濱偶聯藥物在腫瘤組織中富集度更高,能提高療效并減少毒性 [33]。值得關注的是,基質金屬蛋白酶MT1-MMP可裂解EphA2胞外N端,生成促癌片段EphA2-NF [39]。血清EphA2-NF水平在PDC患者中顯著升高,包括早期病例,與EphA2缺失的組織學特征一致 [39]。因此,EphA2-NF可作為一種無創生物標志物,為PDC早期檢測與風險評估提供新思路。
4.1.3 結直腸癌與耐藥機制
在結直腸癌(CRC)中,EphA2高表達與EGFR靶向藥物西妥昔單抗(Cetuximab)耐藥密切相關 [5]。EphA2激活能觸發替代性生存信號,削弱抗EGFR療效。EphA2抑制劑ALW-II-41-27與西妥昔單抗聯合可逆轉耐藥,抑制腫瘤生長并誘導細胞凋亡 [5]。臨床分析亦發現EphA2高表達患者的無進展生存期顯著縮短。另一方面,CEACAM1-L通過下調EphA2及STAT3信號抑制CRC肝轉移 [30],提示EphA2調控腫瘤轉移具有情境依賴性。深入解析EphA2與CEACAM家族的交互作用,將有助于開發克服耐藥的新策略。
4.1.4 卵巢癌與化療敏感性調控
高級別漿液性卵巢癌(HGSOC)常因化療耐藥導致復發。研究顯示,EphA2與FAK共同調控PI3K/AKT和MAPK/ERK信號,是藥物敏感性的重要決定因子 [25]。Brigatinib作為雙重抑制劑,可協同PARP抑制劑(PARPi)增強凋亡,改善治療反應 [25]。此外,鉑類化療可誘導ERK1/2-RSK1/2-EphA2-GPRC5A信號開關,引發適應性耐藥 [40]。RSK抑制劑能阻止EphA2 S897磷酸化并恢復鉑敏感性 [40]。該發現揭示EphA2在化療耐藥形成中的核心地位,也為聯合靶向RSK-EphA2通路提供了新方向。
4.1.5 乳腺癌與外泌體信號調控
EphA2在三陰性乳腺癌(TNBC)中高表達,與侵襲性及預后不良密切相關 [31]。EPA與EphA2抑制劑聯合能通過擾亂膽固醇穩態、抑制ABCA1表達,顯著增強細胞凋亡 [31]。此外,高轉移性乳腺癌細胞釋放的外泌體EphA2可激活內皮細胞AMPK信號,促進血管生成與轉移 [11]。這表明EphA2不僅作為腫瘤內信號分子,也通過外泌體介導細胞間通訊,成為腫瘤微環境重塑的重要因子。
4.2 EphA2在心血管與炎癥性疾病中的作用
除腫瘤外,EphA2在心血管與炎癥疾病中亦發揮調節作用。研究發現,EphA2與ephrinA1在血管平滑肌細胞中共同維持血管穩態,其配體結合可抑制VSMC遷移與增殖 [29]。高同型半胱氨酸血癥患者中,EphA2-PI3K/AKT/NF-κB信號被異常激活,導致內皮屏障功能受損 [43]。在肺部炎癥模型中,EphA2調控LPS誘導的細胞因子釋放及屏障完整性 [17]。此外,EphA2在鼻竇黏膜中參與抗病毒免疫反應,通過調控上皮細胞分泌與屏障穩定性影響慢性鼻炎病程 [18,19]。這些研究拓展了EphA2的功能邊界,顯示其在免疫與血管生物學中的重要作用。
5. EphA2靶向藥物與治療策略進展
EphA2因其在多種疾病中的關鍵作用,成為新興的靶向治療焦點。當前的藥物研究主要集中在小分子抑制劑、抗體藥物偶聯物(ADC)、核酸干預及納米遞送系統等方向。部分在研管線如下表:
| 藥物 | 藥物類型 | 在研適應癥(疾病名) | 在研機構 | 最高研發階段 |
|---|---|---|---|---|
| 瑞戈非尼 | 小分子化藥 | 肝癌 | 肝細胞癌 | 結直腸癌 | 胃腸道間質瘤 | 轉移性結直腸癌等 | Bayer AG | Bristol Myers Squibb Co. | Bayer Yakuhin Ltd. | Merck KGaA | Bayer Pharma AG等 | 批準上市 |
| 達沙替尼 | 小分子化藥 | 加速期慢性骨髄性白血病 | 慢性期費城染色體陽性慢性粒細胞白血病 等 | Bristol Myers Squibb Co. | Accord Healthcare SLU | Bristol-Myers Squibb Pharma EEIG等 | 批準上市 |
| Dasatinib monolauryl sulfate | 小分子化藥 | 腫瘤 | 漢達生技醫藥股份有限公司 | 申請上市 |
| Nuzefatide pevedotin | 多肽偶聯藥物(PDC) | 晚期惡性實體瘤 | 非小細胞肺癌 | 卵巢癌 | 頭頸部鱗狀細胞癌等 | Bicycle Therapeutics Plc | 臨床1/2期 |
| TP53-EphA-2-CAR-DC vaccine(Chinese PLA General Hospital) | CAR-DC | - | 浙江大學 | 中國人民解放軍總醫院 | 臨床1期 |
| Dasatinib nanoparticle formulation(Xspray Pharma AB) | 小分子化藥 | 費城染色體陽性慢性粒細胞白血病 | Xspray Pharma AB | 臨床1期 |
| E-SYNC T Cells(University of California) | CAR-T | EGFR突變膠質母細胞瘤 | 復發性膠質母細胞瘤 | The University of California, San Francisco | 臨床1期 |
| EphA2-targeted CAR-DC(Second Affiliated Hospital, School of Medicine, Zhejiang University) | CAR-DC | 轉移性非小細胞肺癌 | 浙江大學醫學院附屬第二醫院(浙江省第二醫院) | 臨床1期 |
| P30-EPS vaccine(Duke University) | 合成肽疫苗 | 治療性疫苗 | 多形性膠質母細胞瘤 | Duke University | 臨床1期 |
| SC-102 | 多肽偶聯藥物(PDC) | 轉移性實體瘤 | 晚期惡性實體瘤 | 按部位分類的實體瘤 | 天津星聯肽生物科技有限公司 | 臨床1期 |
| siRNA-EphA2-DOPC | siRNA | 卵巢癌 | 胰腺癌 | The University of Texas MD Anderson Cancer Center | 臨床1期 |
| MB-108 | 溶瘤病毒 | 多形性膠質母細胞瘤 | 神經膠質肉瘤 | 復發性惡性膠質瘤 | 膠質母細胞瘤 | The University of Alabama at Birmingham | Nationwide Children's Hospital | Mustang Bio, Inc. | 臨床1期 |
| EphA2-targeted CAR-T Cell(Second Affiliated Hospital, School of Medicine, Zhejiang University) | CAR-T | 轉移性非小細胞肺癌 | 浙江大學醫學院附屬第二醫院(浙江省第二醫院) | 臨床1期 |
(數據截止到2025年11月4日,來源于synapse)
6. EphA2研究工具
EphA2受體酪氨酸激酶作為信號轉導樞紐,在多種腫瘤及炎癥疾病中展現雙重功能。華美生物提供EphA2研究相關重組蛋白、抗體、ELISA試劑盒,助力您關于EphA2信號雙向調控的分子機制研究,或EphA2靶向藥物開發。
● EphA2重組蛋白
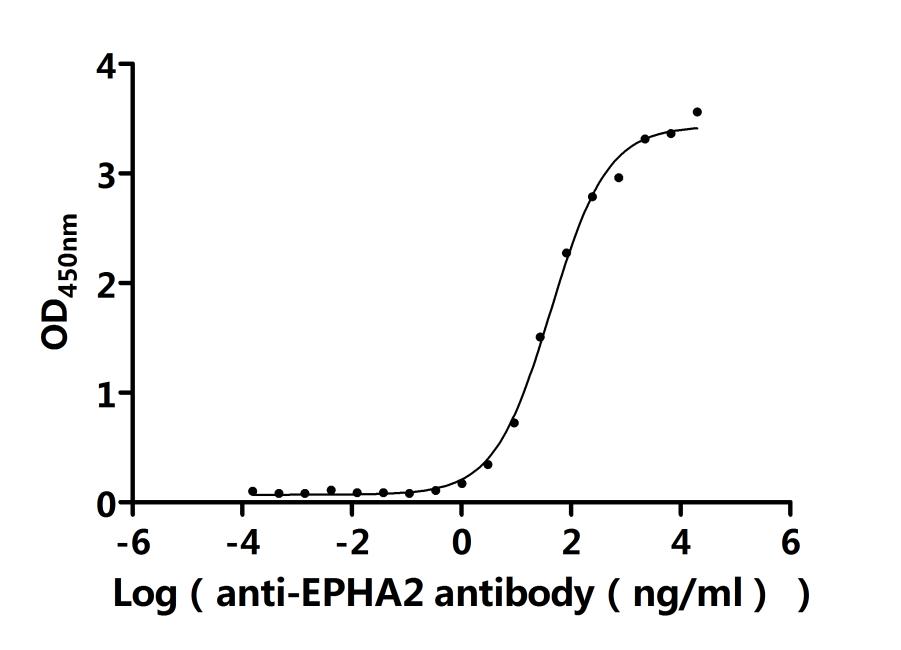
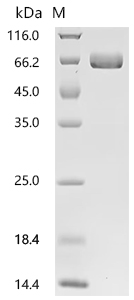
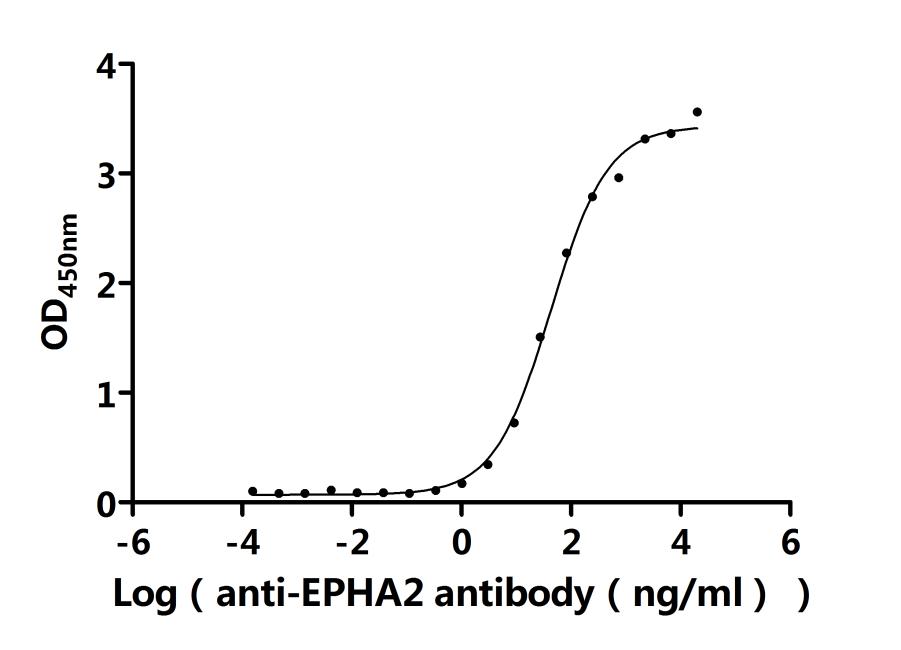
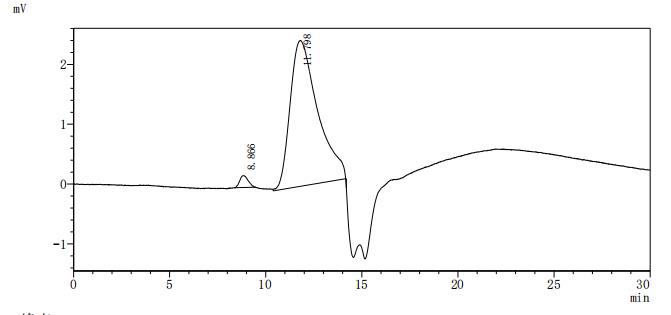
Recombinant Human Ephrin type-A receptor 2 (EPHA2F), partial (Active); CSB-MP007722HUd7
● EphA2抗體
● EphA2 ELISA試劑盒
● EphA2穩轉細胞株
參考文獻:
[1] Yuki Wakayama, K. Miura, H. Sabe, N. Mochizuki.(2011). EphrinA1-EphA2 Signal Induces Compaction and Polarization of Madin-Darby Canine Kidney Cells by Inactivating Ezrin through Negative Regulation of RhoA.
[2] M. Parri, F. Buricchi, M. Taddei, E. Giannoni, G. Raugei, G. Ramponi, P. Chiarugi.(2005). EphrinA1 Repulsive Response Is Regulated by an EphA2 Tyrosine Phosphatase.
[3] Deo R. Singh, Pranjali P Kanvinde, Christopher King, E. Pasquale, K. Hristova.(2018). The EphA2 receptor is activated through induction of distinct, ligand-dependent oligomeric structures.
[4] Xiaoying Huang, Li Na, Qing Han, Qilun Liu, Ligang Wu.(2025). EPHA2 promotes triple-negative breast cancer progression by suppressing pyroptosis via the AKT/PI3K/mTOR pathway.
[5] G. Martini, C. Cardone, P. Vitiello, V. Belli, S. Napolitano, T. Troiani, D. Ciardiello, C. D. Della Corte, F. Morgillo, N. Matrone, V. Sforza, G. Papaccio, V. Desiderio, M. Paul, V. Moreno‐Viedma, N. Normanno, A. Rachiglio, V. Tirino, E. Maiello, T. Latiano, D. Rizzi, G. Signoriello, M. Sibilia, F. Ciardiello, E. Martinelli.(2019). EPHA2 Is a Predictive Biomarker of Resistance and a Potential Therapeutic Target for Improving Antiepidermal Growth Factor Receptor Therapy in Colorectal Cancer.
[6] Ryan Lingerak, A. Petty, Hong Guo, Hebei Lin, Xiaojun Shi, Soyeon Kim, Bingcheng Wang.(2024). Abstract 1680: Roles of EphA2 receptor signaling in prostate cancer development and progression.
[7] Hee-sung Kim, Y. Won, J. Shim, Hyun Ji Kim, B. Kim, H. Hong.(2019). Role of EphA2-PI3K signaling in vasculogenic mimicry induced by cancer-associated fibroblasts in gastric cancer cells.
[8] J. Jukonen, L. Moyano-Galceran, K. H?pfner, E. Pietil?, Laura Lehtinen, K. Huhtinen, E. Gucciardo, J. Hynninen, S. Hietanen, S. Grénman, P. Ojala, O. Carpén, K. Lehti.(2021). Aggressive and recurrent ovarian cancers upregulate ephrinA5, a non-canonical effector of EphA2 signaling duality.
[9] Hayato Kawachi, Tadaaki Yamada, Yuki Katayama, Takayama Koichi.(2025). Abstract 5532: Combinational targeted therapy effects on EphA2 and KRAS G12C inhibitor against KRAS G12C mutated non-small cell lung cancer cells.
[10] H. E, Lei Zhang, Zhenhua Yang, Long Xu, Tao Wang, Junhong Guo, Lang Xia, Juemin Yu, Heyong Wang, Y. She, Junqi Wu, Yue Zhao, Chang Chen, Deping Zhao.(2024). SNAI1 promotes epithelial-mesenchymal transition and maintains cancer stem cell-like properties in thymic epithelial tumors through the PIK3R2/p-EphA2 Axis.
[11] B. Han, He Zhang, Ruinan Tian, Hui Liu, Zhaosong Wang, Zhiyong Wang, Jianfei Tian, Yanfen Cui, Sixin Ren, Xiaoyan Zuo, Ran Tian, R. Niu, Fei Zhang.(2022). Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling.
[12] Zicong Gao, Xingxing Han, Yuying Zhu, He Zhang, Ran Tian, Zhiyong Wang, Yanfen Cui, Zhaosong Wang, R. Niu, Fei Zhang.(2020). Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling.
[13] Yunyun Wang, Yong Liu, Guo Li, Z. Su, Shuling Ren, Ping-qing Tan, Xin Zhang, Y. Qiu, Yong-quan Tian.(2014). Ephrin type-A receptor 2 regulates sensitivity to paclitaxel in nasopharyngeal carcinoma via the phosphoinositide 3-kinase/Akt signalling pathway.
[14] Fang Zhang, Yue Zhou, Naru Hamada, Akihiro Tanaka, Satoru Yokoyama, Seiji Yano, Kunio Matsumoto, Hiroyuki Mano, Hiroaki Sakurai.(2025). Stress Response Kinase MK2 Induces Non-canonical Activation of EphA2 in EML4-ALK Lung Cancer Cells.
[15] Ryan Lingerak, Bingcheng Wang.(2023). Abstract B018: Loss of androgen receptor-mediated repression leads to EphA2 overexpression that promotes cellular dedifferentiation and castration resistance of prostate cancer through noncanonical signaling.
[16] Yi-Ping Xiang, T. Xiao, Qi-Guang Li, Shan-Shan Lu, Wei Zhu, Yun-Ya Liu, Jie-Ya Qiu, Z. Song, Wei Huang, Hong Yi, Yao-Yun Tang, Zhi-Qiang Xiao.(2020). Y772 phosphorylation of EphA2 is responsible for EphA2-dependent NPC nasopharyngeal carcinoma growth by Shp2/Erk-1/2 signaling pathway.
[17] J. Hong, M. H. Shin, K. Chung, E. Y. Kim, J. Jung, Y. Kang, Y. S. Kim, S. K. Kim, Joon Chang, M. Park.(2015). EphA2 Receptor Signaling Mediates Inflammatory Responses in Lipopolysaccharide-Induced Lung Injury.
[18] J. Shin, M. Han, Jae Hyung Park, Seung Hyeok Lee, Tae Hoon Kim, Sang Hag Lee.(2023). The EphA1 and EphA2 Signaling Modulates the Epithelial Permeability in Human Sinonasal Epithelial Cells and the Rhinovirus Infection Induces Epithelial Barrier Dysfunction via EphA2 Receptor Signaling.
[19] Sang Hag Lee, Sung Hoon Kang, M. Han, Jinsook Kwak, H. Kim, Tae Hoon Lee, Dabin Lee, Tae Hoon Kim.(2021). The Expression of ephrinA1/ephA2 Receptor Increases in Chronic Rhinosinusitis and ephrinA1/ephA2 Signaling Affects Rhinovirus-Induced Innate Immunity in Human Sinonasal Epithelial Cells.
[20] Elisa Fontana, Babar Bashir, Judy S. Wang, R. Aljumaily, J. Machiels, M. Vieito, G. Falchook, Louise Carter, B. D. de Spéville, A. Greystoke, Sang Wun Kim, Nuria Kotecki, A. Spira, I. M. Candilejo, Bristi Basu, H. Prenen, A. Bessudo, Misako Nagasaka, J. Ahnert, Joo-Hwan Park, M. Teo, J. Rotow, Jie Liu, Assunta De Rienzo, Mengyao Li, Adriana Domingo, Hanna Orr, G. Bennett, Rajiv Sharma, M. McKean.(2023). Abstract LB_A17: Trial in progress: First-in-human phase I dose-escalation study of a novel Bicycle toxin conjugate (BT5528) targeting EphA2 in patients with advanced solid tumors.
[21] Alix Tr?ster, Michael Diprima, Nathalie Jores, D. Kudlinzki, S. Sreeramulu, S. Gande, Verena Linhard, Damian Ludig, Alexander Schug, K. Saxena, Maria Reinecke, S. Heinzlmeir, M. Leisegang, J. Wollenhaupt, Frank Lennartz, M. Weiss, B. Kuster, G. Tosato, H. Schwalbe.(2023). Optimization of the Lead Compound NVP-BHG712 as Colorectal Cancer Inhibitor.
[22] Yige Fu, Drishti Rathod, Ehab M. Abo-Ali, V. Dukhande, Ketan Patel.(2019). EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma.
[23] Jia-Lin Wang, Yu-ling Liu, Y. Li, Wenbing Dai, Zhao-ming Guo, Zhao-hui Wang, Qiang Zhang.(2012). EphA2 targeted doxorubicin stealth liposomes as a therapy system for choroidal neovascularization in rats.
[24] Xia Li, Dan Li, Rong Ma.(2022). ALW-II-41-27, an EphA2 inhibitor, inhibits proliferation, migration and invasion of cervical cancer cells via inhibition of the RhoA/ROCK pathway.
[25] Julie R. Duffield, Xiaonan Hou, Benjamin W. Wilson, Anjali Prasad, Iman K. McKeon-Makki, A. Huehls, Xinyan Wu, Cristina Correia, Melissa C. Larson, F. Couch, A. Oberg, Scott H. Kaufmann, L. Karnitz, Arun Kanakkanthara.(2025). Abstract A024: A PARP-inhibitor-induced early adaptive survival response is tackled through depletion of FRA1 by brigatinib in high-grade serous ovarian carcinoma.
[26] R. Joseph, S. Dasari, Sujanitha Umamaheswaran, L. Mangala, E. Bayraktar, Cristian Rodríguez-Aguayo, Yutuan Wu, N. Nguyen, Reid T Powell, Mary Sobieski, Yuan Liu, Mark S Kim, Sara Corvigno, Katherine I Foster, Pahul Hanjra, Thanh Chung Vu, Mamur A. Chowdhury, P. Amero, Clifford Stephan, G. Lopez-Berestein, S. Westin, Anil K. Sood.(2024). EphA2- and HDAC-Targeted Combination Therapy in Endometrial Cancer.
[27] Zhongwen Chen, D. Oh, K. Biswas, Cheng-han Yu, R. Zaidel-Bar, J. Groves.(2018). Spatially modulated ephrinA1:EphA2 signaling increases local contractility and global focal adhesion dynamics to promote cell motility.
[28] Ryan J Schuck, Alyssa E Ward, A. Sahoo, Jennifer A. Rybak, Robert J Pyron, Thomas N Trybala, Timothy B Simmons, Joshua A. Baccile, Ioannis Sgouralis, Matthias Buck, Rajan Lamichhane, Francisco N. Barrera.(2025). Cholesterol inhibits assembly and oncogenic activation of the EphA2 receptor.
[29] Matthew L. Scott, Alexandra C. Finney, Wayne W Orr.(2023). Abstract 705: Epha2 Ligand-dependent And Ligand-independent Signaling In Vascular Smooth Muscle Cell Proliferation And Migration.
[30] A. Arabzadeh, Kevin McGregor, Valérie Breton, L. Van Der Kraak, U. Akavia, C. Greenwood, N. Beauchemin.(2017). EphA2 signaling is impacted by carcinoembryonic antigen cell adhesion molecule 1-L expression in colorectal cancer liver metastasis in a cell context-dependent manner.
[31] Angie M. Torres-Adorno, H. Vitrac, Y. Qi, L. Tan, K. Levental, Yang-Yi Fan, Peiying Yang, R. Chapkin, Bedrich L Eckhardt, N. Ueno.(2018). Eicosapentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux.
[32] Dominique V. Parker, Verra M. Ngwa, Jin Chen, Julie A. Rhoades.(2024). Abstract 6866: Investigating the role of Epha2 in breast cancer-mediated myeloid cell expansion and function.
[33] B. Quinn, Si Wang, E. Barile, Swadesh K. Das, L. Emdad, D. Sarkar, S. De, Susan Morvaridi Kharagh, J. Stebbins, S. Pandol, P. Fisher, M. Pellecchia.(2016). Therapy of pancreatic cancer via an EphA2 receptor-targeted delivery of gemcitabine.
[34] Jie-Yu Tang, Yun-Xi Peng, Wei Zhu, Jie-Ya Qiu, Wei Huang, Hong Yi, Shan-Shan Lu, Juan Feng, Zheng-Zheng Yu, Di Wu, Qi Wen, Li Yuan, Jinwu Peng, Zhi-Qiang Xiao.(2025). USP5 Binds and Stabilizes EphA2 to Increase Nasopharyngeal Carcinoma Radioresistance.
[35] Hui-Hua Zhou, Jinfeng Zhao, Xiaolin Yang, Jie Liu, Wei Huang.(2022). Study on the Expression of β-1,3-N-acetylglucosaminyltransferase 3 in Gastric Cancer and the Mechanism Promoting Gastric Cancer Progression Based on the Extraction Method of Nanomagnetic Beads.
[36] Nobuhiko Asakura, Naotoshi Nakamura, Atsushi Muroi, Yosui Nojima, T. Yamashita, S. Kaneko, Kazuki Ikeda, N. Koshikawa, Takashi Suzuki.(2021). Expression of Cancer Stem Cell Markers EpCAM and CD90 Is Correlated with Anti- and Pro-Oncogenic EphA2 Signaling in Hepatocellular Carcinoma.
[37] Zhongwen Chen, D. Oh, K. Biswas, R. Zaidel-Bar, J. Groves.(2021). Probing the effect of clustering on EphA2 receptor signaling efficiency by subcellular control of ligand-receptor mobility.
[38] Carolin Offenh?user, K. Dave, K. Beckett, Fiona M. Smith, Buddhika A. Jayakody, Leanne T. Cooper, Helen Agyei-Yeboah, Jennifer K. McCarron, Yuchen Li, Kate Bastick, F. Al-Ejeh, Jason K. Cullen, M.
Coulthard, Jeffrey J. Gorman, Andrew W. Boyd, B. Day.(2024). EphA2 regulates vascular permeability and prostate cancer metastasis via modulation of cell junction protein phosphorylation.[39] N. Koshikawa, Shinya Sato, K. Nio, T. Terashima, M. Ueno, Taro Yamashita.(2024). Abstract A059: Serum EphA2 proteolytic fragment is a potent biomarker for diagnosing a very early stage of ductal pancreatic carcinoma.
[40] L. Moyano-Galceran, E. Pietil?, S. Turunen, Sara Corvigno, E. Hjerpe, Daria R Bulanova, U. Joneborg, Twana Alkasalias, Yuichiro Miki, M. Yashiro, A. Chernenko, J. Jukonen, Madhurendra Singh, Hanna Dahlstrand, J. Carlson, K. Lehti.(2020). Adaptive RSK‐EphA2‐GPRC5A signaling switch triggers chemotherapy resistance in ovarian cancer.
[41] Xinxin Gan, Jiatao Hu, Qingyang Pang, Rui Yan, Y. Bao, Ying Liu, Jiaao Song, Zheng Wang, Weihao Sun, Fuzhao Huang, Chen Cai, Linhui Wang.(2024). LDHA‐mediated M2‐type macrophage polarization via tumor‐derived exosomal EPHA2 promotes renal cell carcinoma progression.
[42] Anan Li, Shijiang Wang, Jiangbo Nie, Shining Xiao, Xinsheng Xie, Yu Zhang, Weilai Tong, Geliang Yao, Ning Liu, Fan Dan, Zhiguo Shu, Jiaming Liu, Zhili Liu, Feng Yang.(2024). USP3 promotes osteosarcoma progression via deubiquitinating EPHA2 and activating the PI3K/AKT signaling pathway.
[43] Dan Tian, Q. Qin, Mingfei Li, Xiaoyu Li, Qing Xu, Qian-zhou Lv.(2021). Homocysteine Impairs Endothelial Cell Barrier Function and Angiogenic Potential via the Progranulin/EphA2 Pathway.
[44] Yidan Wang, Zhenting Zhang, Zhengyan Zhu, P. Wang, Jinjuan Zhang, Hui Liu, Jianyu Li.(2022). The significance of EphA2-regulated Wnt/β-catenin signal pathway in promoting the metastasis of HBV-related hepatocellular carcinoma.
[45] Qiong Wang, Xiangyi Kong, Hongming Song, Li Wang, Lingrui Li, Xiaonan Hou, S. Renuse, M. S. Zahari, Ran Cheng, Md Kamrul Hasan Khan, Jidong Wang, Kiran K. Mangalaparthi, Lin Fang, T. Lotan, B. H. Park, S. Weroha, Huaijun Zhou, Akhilesh Pandey, Xinyan Wu.(2025). Proteomic Analysis of PTEN-Deficient Cells Reveals Src-Mediated Upregulation of EphA2 and Therapeutic Potential of Dual Inhibition.
[46] C. Allocca, A. Cirafici, M. Laukkanen, M. Castellone.(2020). Serine 897 Phosphorylation of EPHA2 Is Involved in Signaling of Oncogenic ERK1/2 Drivers in Thyroid Cancer Cells.