乙酰膽堿受體α1亞基(CHRNA1):結構、功能機制及相關疾病研究進展
日期:2025-10-14 09:15:16
1. CHRNA1的背景介紹
1.1 CHRNA1的分子結構與功能特性
CHRNA1基因位于人類2號染色體q31.1,編碼煙堿型乙酰膽堿受體(nAChR)的α1亞基。該蛋白含457個氨基酸,具備四個跨膜結構域(M1–M4),其中M2形成離子通道孔道,負責陽離子通透 [1]。在神經肌肉接頭(NMJ),CHRNA1與β1、δ、ε/γ亞基組裝為五聚體受體,介導乙酰膽堿(ACh)信號,引發肌細胞膜去極化與肌肉收縮 [2][3]。
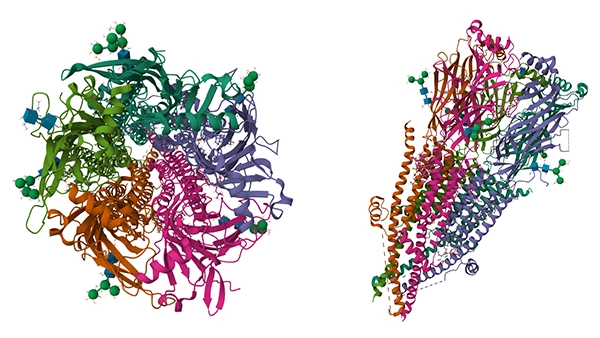
Human muscle nAChR apo state (圖源:PDB)
基因敲除實驗表明,CHRNA1缺失會導致NMJ突觸后膜發育異常并阻斷信號傳遞,突顯其在突觸形成和功能維持中的關鍵作用 [2]。此外,CHRNA1胞內環區可與rapsyn結合,確保受體在突觸后膜高密度聚集 [4]。其M3-M4連接區的磷酸化修飾還可調控受體內化速率,對突觸可塑性具有重要意義 [3]。成人骨骼肌中,ε亞基替代胎兒γ亞基,提高通道電導率,該亞基轉換依賴agrin-MuSK信號通路調控 [5]。
1.2 CHRNA1的表達分布
CHRNA1在外周神經系統NMJ終板區高表達,并與乙酰膽堿酯酶共定位,形成高效的突觸后致密區 [6][7]。在中樞神經系統(如海馬、皮層、腦干),其表達較低,但提示可能參與認知調節和自主神經功能 [8]。
胸腺髓質上皮細胞中CHRNA1的“異位表達”受AIRE調控,與自身免疫耐受及重癥肌無力相關 [6]。單細胞測序顯示,CHRNA1在脊髓前角運動神經元中呈階段性高峰,與NMJ發育同步 [9]。此外,在肺動脈平滑肌細胞中也可檢測到CHRNA1轉錄本,其水平與線粒體復合物Ⅰ活性呈負相關,可能參與血管張力調控 [10]。
1.3 CHRNA1與相關受體的相互作用
CHRNA1作為nAChR的核心亞基,其N端胞外區含有ACh結合位點,M2跨膜螺旋形成離子通道 [5]。研究表明,CHRNA1與β1的相互作用可影響通道開放概率;當β1突變時,電流幅度下降約60% [11]。胚胎期CHRNA1-γ受體被ε亞基替代后,通道動力學特性明顯改變 [1]。
CHRNA1還能與α7或α3β4等亞基形成異源受體,改變鈣離子通透性及脫敏速率 [12]。此外,CHRNA1可與5-HT3A、GABAA等受體存在交叉調控,影響電流衰減和受體敏感性 [11][2]。這些互作在病理條件下可能受損,如慢阻肺患者中CHRNA1與α9共定位消失并伴隨炎癥因子異常升高 [11]。
2. CHRNA1的作用機制與信號通路
2.1 突觸信號傳遞機制
當運動神經元釋放ACh時,兩個ACh分子與CHRNA1結合,觸發通道開放,產生終板電位并激活電壓門控鈉通道,引發肌肉收縮 [5]。其在突觸后膜的聚集依賴agrin-LRP4-MuSK-rapsyn通路 [5]。在MuSK基因敲除小鼠中,CHRNA1水平下降80%,突觸褶皺消失 [5]。
此外,PKA介導的磷酸化可增強受體敏感性,CaMKII則加速脫敏 [13]。在轉錄水平,neuregulin-1和MuSK信號促進CHRNA1表達及亞基轉換。基因多態性(如rs16862847)與終板電位下降相關 [12]。在慢阻肺患者中,CHRNA1表達下調與氣道收縮障礙相關 [11]。
2.2 與MuSK通路的關聯
CHRNA1在agrin-MuSK通路中發揮核心作用。LRP4突變會削弱MuSK磷酸化及CHRNA1定位,導致先天性肌無力綜合征(CMS)[3]。抗MuSK抗體同樣可影響CHRNA1在突觸膜的錨定 [1]。此外,CHRNA1表達變化還可能通過mTOR通路影響胸腺免疫耐受,提示其在神經-免疫交叉調控中的作用 [5]。
2.3 在mTOR通路中的調控
胸腺上皮細胞中,CHRNA1通過mTOR通路調控免疫耐受。其缺失會降低mTOR活性,導致胸腺發育障礙及自身免疫反應 [9][14]。CHRNA1與STAT3互作可激活Akt/mTOR通路,在尼古丁誘導的動脈粥樣硬化模型中影響免疫功能 [15]。CHRNA1-mTOR軸過度激活可能與自身免疫性重癥肌無力相關 [7]。
3. CHRNA1相關疾病
3.1 先天性肌無力綜合征(CMS)
CHRNA1突變約占CMS的10%–15%,多為常染色體隱性遺傳 [1]。錯義和無義突變可降低受體對ACh的敏感性或表達水平,導致終板電位顯著下降 [5]。患者常在嬰兒期發病,表現為眼瞼下垂、喂養困難、全身肌無力,且對吡啶斯的明反應有限 [16]。部分突變(如V285L)引起溫度敏感性肌無力,機制可能與突變蛋白熱不穩定性相關 [1]。
3.2 肺動脈高壓(PAH)
CHRNA1可能通過調控線粒體電子傳遞鏈功能及ROS生成參與PAH。其在PAH患者肺組織中高表達,并與ETC復合物Ⅱ-Ⅲ活性降低相關 [10]。HIF-1α可上調CHRNA1轉錄 [11]。敲低CHRNA1可改善平滑肌細胞代謝異常與凋亡抵抗 [10]。動物模型顯示,CHRNA1拮抗劑可降低肺動脈壓力升高 [10]。
3.3 骨關節炎(OA)
OA患者滑膜中CHRNA1 mRNA顯著上調,并與炎癥因子IL-6、TNF-α正相關 [2]。多組學研究提示腸道菌群可通過“腸-肌肉-關節軸”調控CHRNA1表達,影響關節穩態 [17]。CHRNA1異常還與自噬障礙、線粒體損傷及軟骨退變相關 [12][17]。其基因多態性與OA疼痛敏感性及病程進展有關 [8]。
4. CHRNA1靶點藥物的最新研究進展
在疾病治療方面,已有多種圍繞CHRNA1的策略被提出。針對原發性局灶性多汗癥,研究提示PAI1能夠抑制CHRNA1表達,從而減少汗腺分泌;相反,其抑制劑PAI-039或PAI1基因敲除會增強與CHRNA1相關的多汗表型。而CHRNA1拮抗劑順式阿曲庫銨則可通過阻斷離子通道緩解多汗癥狀。
對于CHRNA1基因突變導致的先天性肌無力綜合征(CMS),靶向外顯子P3A 5′剪接位點的反義寡核苷酸(AONs)被證明能夠恢復正常剪接平衡,顯示出潛在的治療前景。此外,CHRNA1基因多態性還會影響患者對羅庫溴銨等肌松藥的敏感性及不良反應風險,為個體化用藥提供了遺傳學依據。
5. 華美生物CHRNA1研究相關產品
CHRNA1基因編碼的乙酰膽堿受體α1亞基不僅是神經肌肉接頭信號傳遞的核心分子,還通過與MuSK和mTOR通路的互作參與免疫調控和代謝調節。其突變或異常表達已被證實與CMS、PAH及OA等疾病密切相關。
華美生物提供多種CHRNA1相關蛋白和抗體產品,支持科研人員在分子機制研究和靶向治療探索中的應用,助力推動CHRNA1在基礎研究與臨床轉化中的進一步發展。
參考文獻:
[1] Pedro M. Rodríguez Cruz, Jacqueline Palace, David Beeson. Congenital myasthenic syndromes and the neuromuscular junction[J]. Current Opinion in Neurology, 2014, 27(5): 566-575.
[2] Qin Chen, Shaosheng Bei, Zhiyun Zhang, Xiaofeng Wang, Yunying Zhu. Identification of diagnostic biomarks and immune cell infiltration in ulcerative colitis[J]. Scientific Reports, 2023, 13(1).
[3] Bisei Ohkawara, Macarena Cabrera‐Serrano, Tomohiko Nakata, Margherita Milone, Nobuyuki Asai, Kenyu Ito, Mikako Ito, Akio Masuda, Yasutomo Ito, Andrew G. Engel, Kinji Ohno. LRP4 third β-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner[J]. Human Molecular Genetics, 2013, 23(7): 1856-1868.
[4] Kai Qian, Jiaxin Xu, Yi Deng, Hao Peng, Jun Peng, Chun‐Mei Ou, Liu Zu, Lihong Jiang, Yonghang Tai. Signaling pathways of genetic variants and miRNAs in the pathogenesis of myasthenia gravis[J]. Gland Surgery, 2020, 9(6): 1933-1944.
[5] Bisei Ohkawara, Mikako Ito, Kinji Ohno. Secreted Signaling Molecules at the Neuromuscular Junction in Physiology and Pathology[J]. International Journal of Molecular Sciences, 2021, 22(5): 2455-2455.
[6] Matthieu Giraud, Richard Taubert, Claire Vandiedonck, Xiayi Ke, Matthieu Lévi‐Strauss, Franco Pagani, Francisco E. Baralle, B. Eymard, Christine Tranchant, Philippe Gajdos, Angela Vincent, Nick Willcox, David Beeson, Bruno Kyewski, Henri-Jean Garchon. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus[J]. Nature, 2007, 448(7156): 934-937.
[7] Matthieu Giraud, Claire Vandiedonck, Henri‐Jean Garchon. Genetic Factors in Autoimmune Myasthenia Gravis[J]. Annals of the New York Academy of Sciences, 2008, 1132(1): 180-192.
[8] Peter Mu‐Hsin Chang, Yi‐Chen Yeh, Tzu-Chi Chen, Yu–Chung Wu, Pei‐Jung Lu, Hui-Chuan Cheng, Hsueh‐Ju Lu, Ming‐Huang Chen, Teh‐Ying Chou, Chi‐Ying F. Huang. High Expression of CHRNA1 is Associated with Reduced Survival in Early Stage Lung Adenocarcinoma after Complete Resection[J]. Annals of Surgical Oncology, 2013, 20(11): 3648-3654.
[9] Zhanfeng Liang, Lianjun Zhang, Huiting Su, Rong Luan, Ning Na, Lina Sun, Yang Zhao, Xiaodong Zhang, Qian Zhang, Juan Li, Lianfeng Zhang, Yong Zhao. MTOR signaling is essential for the development of thymic epithelial cells and the induction of central immune tolerance[J]. Autophagy, 2017, 14(3): 505-517.
[10] Xin Zhang, Jieling Li, Minyi Fu, Xijie Geng, Junjie Hu, Kejing Tang, Pan Chen, Jianyong Zou, Xiaoman Liu, Bo Zeng. Dysfunction in mitochondrial electron transport chain drives the pathogenesis of pulmonary arterial hypertension: insights from a multi-omics investigation[J]. Respiratory Research, 2025, 26(1).
[11] Lin Chen, Donglan Zhu, Jinfu Huang, Hui Zhang, Guang Zhou, Xiaoning Zhong. Identification of Hub Genes Associated with COPD Through Integrated Bioinformatics Analysis[J]. International Journal of COPD, 2022, Volume 17: 439-456.
[12] Patrick F. McArdle, Sue Rutherford, Braxton D. Mitchell, Coleen Damcott, Ying Wang, Ramachandran S. Vasan, Sandy Ott, Yen‐Pei C. Chang, Daniel Levy, Nanette Steinle. Nicotinic acetylcholine receptor subunit variants are associated with blood pressure
[13] Peter V. Lovell, David F. Clayton, Kirstin Replogle, Claudio V. Mello. Birdsong "Transcriptomics": Neurochemical Specializations of the Oscine Song System[J]. PLoS ONE, 2008, 3(10): e3440-e3440.
[14] Fatemeh Shirafkan, Luca Hensel, Kristin Rattay. Immune tolerance and the prevention of autoimmune diseases essentially depend on thymic tissue homeostasis[J]. Frontiers in Immunology, 2024, 15.
[15] Shuang Xu, Huaner Ni, Hangwei Chen, Qiuyan Dai. The interaction between STAT3 and nAChRα1 interferes with nicotine-induced atherosclerosis via Akt/mTOR signaling cascade[J]. Aging, 2019, 11(19): 8120-8138.
[16] Marco Calabrò, Laura Mandelli, Concetta Crisafulli, Antonella Sidoti, Tae‐Youn Jun, Soo-Jung Lee, Hans‐Jürgen M?ller, Ashwin A. Patkar, Prakash S. Masand, Chi‐Un Pae, Alessandro Serretti. Genes Involved in Neurodevelopment, Neuroplasticity, and Bipolar Disorder: CACNA1C, CHRNA1, and MAPK1[J]. Neuropsychobiology, 2016, 74(3): 159-168.
[17] Tianyang Xu, Dong Kwon Yang, Kaiyuan Liu, Qiuming Gao, Zhongchen Liu, Guodong Li. Miya Improves Osteoarthritis Characteristics via the Gut-Muscle-Joint Axis According to Multi-Omics Analyses[J]. Frontiers in Pharmacology, 2022, 13.