Recombinant Human Nuclear autoantigen Sp-100 (SP100), partial
-
中文名稱:人SP100重組蛋白
-
貨號:CSB-YP022439HU
-
規格:
-
來源:Yeast
-
其他:
-
中文名稱:人SP100重組蛋白
-
貨號:CSB-EP022439HU
-
規格:
-
來源:E.coli
-
其他:
-
中文名稱:人SP100重組蛋白
-
貨號:CSB-EP022439HU-B
-
規格:
-
來源:E.coli
-
共軛:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名稱:人SP100重組蛋白
-
貨號:CSB-BP022439HU
-
規格:
-
來源:Baculovirus
-
其他:
-
中文名稱:人SP100重組蛋白
-
貨號:CSB-MP022439HU
-
規格:
-
來源:Mammalian cell
-
其他:
產品詳情
-
純度:>85% (SDS-PAGE)
-
基因名:SP100
-
Uniprot No.:
-
別名:DKFZp686E07254; FLJ00340; FLJ34579; Lysp100b; Nuclear antigen Sp100; Nuclear autoantigen Sp 100; Nuclear autoantigen Sp-100; Nuclear autoantigen Sp100; Nuclear dot associated Sp100 protein; Nuclear dot-associated Sp100 protein; SP 100; SP100; SP100 HMG nuclear autoantigen; SP100 nuclear antigen; SP100_HUMAN; Speckled 100 kDa
-
種屬:Homo sapiens (Human)
-
蛋白長度:Partial
-
蛋白標簽:Tag?type?will?be?determined?during?the?manufacturing?process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
產品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
復溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
儲存條件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保質期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
貨期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事項:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相關產品
靶點詳情
-
功能:Together with PML, this tumor suppressor is a major constituent of the PML bodies, a subnuclear organelle involved in a large number of physiological processes including cell growth, differentiation and apoptosis. Functions as a transcriptional coactivator of ETS1 and ETS2 according to PubMed:11909962. Under certain conditions, it may also act as a corepressor of ETS1 preventing its binding to DNA according to PubMed:15247905. Through the regulation of ETS1 it may play a role in angiogenesis, controlling endothelial cell motility and invasion. Through interaction with the MRN complex it may be involved in the regulation of telomeres lengthening. May also regulate TP53-mediated transcription and through CASP8AP2, regulate FAS-mediated apoptosis. Also plays a role in infection by viruses, including human cytomegalovirus and Epstein-Barr virus, through mechanisms that may involve chromatin and/or transcriptional regulation.
-
基因功能參考文獻:
- the ND10 bodies become viral replication compartments, and ICP0, a viral E3 ligase, degrades both PML and SP100. The amounts of PML and SP100 and the number of ND10 structures increase in cells exposed to IFN-beta. PMID: 28439026
- that Sp100 represses viral transcription and replication in differentiated cells PMID: 28968443
- Data suggest that nuclear antigen Sp100C is a multifaceted histone H3 methylation and phosphorylation sensor. PMID: 27129259
- These results suggest that high-risk human papillomavirus 31 target interferon kappa to prevent Sp100 expression and identify Sp100 as an interferon-stimulated gene with anti-human papillomavirus activity. PMID: 26491169
- PML, hDaxx and Sp100 primarily act as cellular restriction factors during lytic human cytomegalovirus replication and during the dynamic process of reactivation but do not serve as key determinants for the establishment of latency. PMID: 26057166
- Sp100 repressed viral transcription and replication only during the initial stages of viral establishment, suggesting that Sp100 acts as a repressor of incoming human papillomavirus type 18 DNA. PMID: 24194542
- Sp100 depletion promotes Adenovirus progeny production and early viral protein synthesis. PMID: 24623443
- Two regions within the N-terminal of the herpes simplex virus 1 ICP0 facilitate the degradation and dissociation of host PML and dissociation of Sp100 from ND10. PMID: 24089549
- Sp100 is recruited to activated arrays in cells expressing the herpes simplex virus type 1 E3 ubiquitin ligase, ICP0, which degrades all Sp100 isoforms except unsumoylated Sp100A. PMID: 23485562
- The results suggest that hantavirus infection interferes with DAXX-mediated apoptosis, and expression of interferon-activated Sp100 and ISG-20 proteins may indicate intracellular intrinsic antiviral attempts. PMID: 23830076
- SP100 and Adeno-associated virus 2 Rep78 are both located in the nucleolus, which provides the spatial possibility for their interaction. PMID: 22419217
- Authors conclude that several ND10 components, including Daxx, the promyelocytic leukemia (PML) protein, and Sp100 cooperate in an additive manner to regulate herpes simplex virus type 1 and human cytomegalovirus infection. PMID: 23221561
- Herpesvirus saimiri tegument protein specifically degraded the cellular ND10 component Sp100. PMID: 22278248
- These findings expand our knowledge of both Sp100 and Cdc20 as well as their role in ubiquitination. PMID: 22086178
- These findings indicate that thehuman herpesvirus 5 IE1-dependent loss of human Sp100 proteins during virus infection may represent an important requirement for efficient viral growth. PMID: 21880768
- Taken together, these data provide evidence that Sp100 is the first ND10-related factor identified that not only possesses the potential to restrict the initial stage of infection but also inhibits cytomegalovirus replication during the late phase. PMID: 21734036
- Sp100 counteract human cytomegalovirus infection via the repression of viral immediate-early gene expression. PMID: 21471311
- SP100 expression reduces malignancy of brain tumors PMID: 21274506
- Endogenous Sp100 may interact with PhiC31 integrase and inhibit the efficiency of PhiC31 integrase-mediated recombination. PMID: 21383994
- Sp100 has a role in the initiation and progression of tumorgenesis PMID: 20512085
- During interphase PML-NBs adopt a spherical organization characterized by the assembly of PML and Sp100 proteins into patches within a 50- to 100-nm-thick shell. PMID: 20130140
- Sp100 interacts with ETS-1 and stimulates its transcriptional activity. PMID: 11909962
- a novel function for Sp100 as a coactivator for HIPK2-mediated p53 activation. PMID: 14647468
- SP100 modulates ETS1-dependent biological processes PMID: 15247905
- Genes that are negatively regulated by ETS1 and upregulated by SP100 have antimigratory or antiangiogenic properties. PMID: 15592518
- EBNA-LP interacts with the promyelocytic leukemia nuclear body (PML NB)-associated protein Sp100 and displaces Sp100 and heterochromatin protein 1alpha (HP1alpha) from PML NBs. PMID: 16177824
- Repressive Sp100 isoforms B, C, and HMG are an essential part of the IFN-beta-mediated suppression of ICP0 expression. PMID: 16873258
- Sp100 isoforms suppress immediate-early HSV-1 proteins at the promoter level and that IFN changes the splicing pattern of the Sp100 transcript to the suppressing Sp100C isoform. PMID: 19279115
顯示更多
收起更多
-
亞細胞定位:Nucleus. Nucleus, PML body. Cytoplasm. Note=Differences in the subnuclear localization of the different isoforms seem to exist and may also be cell cycle- and interferon-dependent. Accumulates in the cytoplasm upon FAS activation.; [Isoform Sp100-C]: Nucleus. Note=Forms a reticulate or track-like nuclear pattern with denser concentrations at the nuclear lamina and surrounding the nucleoli, a pattern reminiscent of heterochromatin-rich regions according to PubMed:11313457.
-
組織特異性:Widely expressed. Sp100-B is expressed only in spleen, tonsil, thymus, mature B-cell line and some T-cell line, but not in brain, liver, muscle or non-lymphoid cell lines.
-
數據庫鏈接:
Most popular with customers
-
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
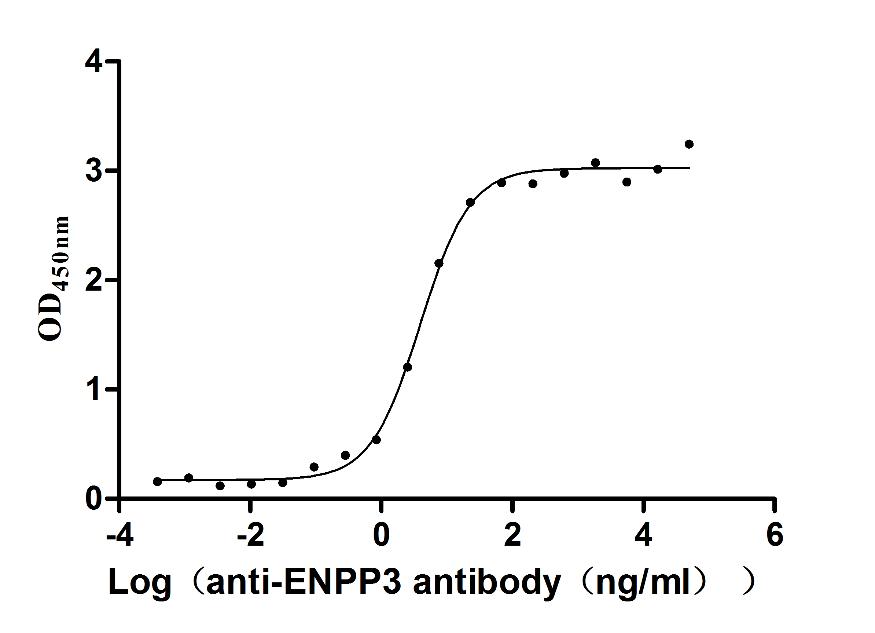
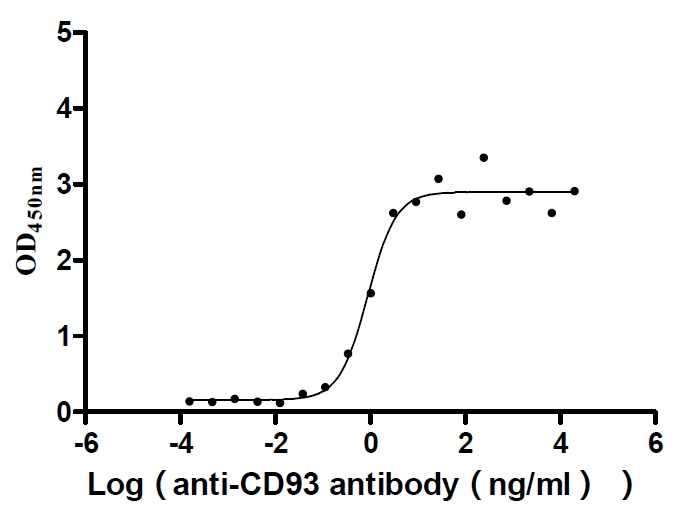
Recombinant Human Complement component C1q receptor (CD93), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
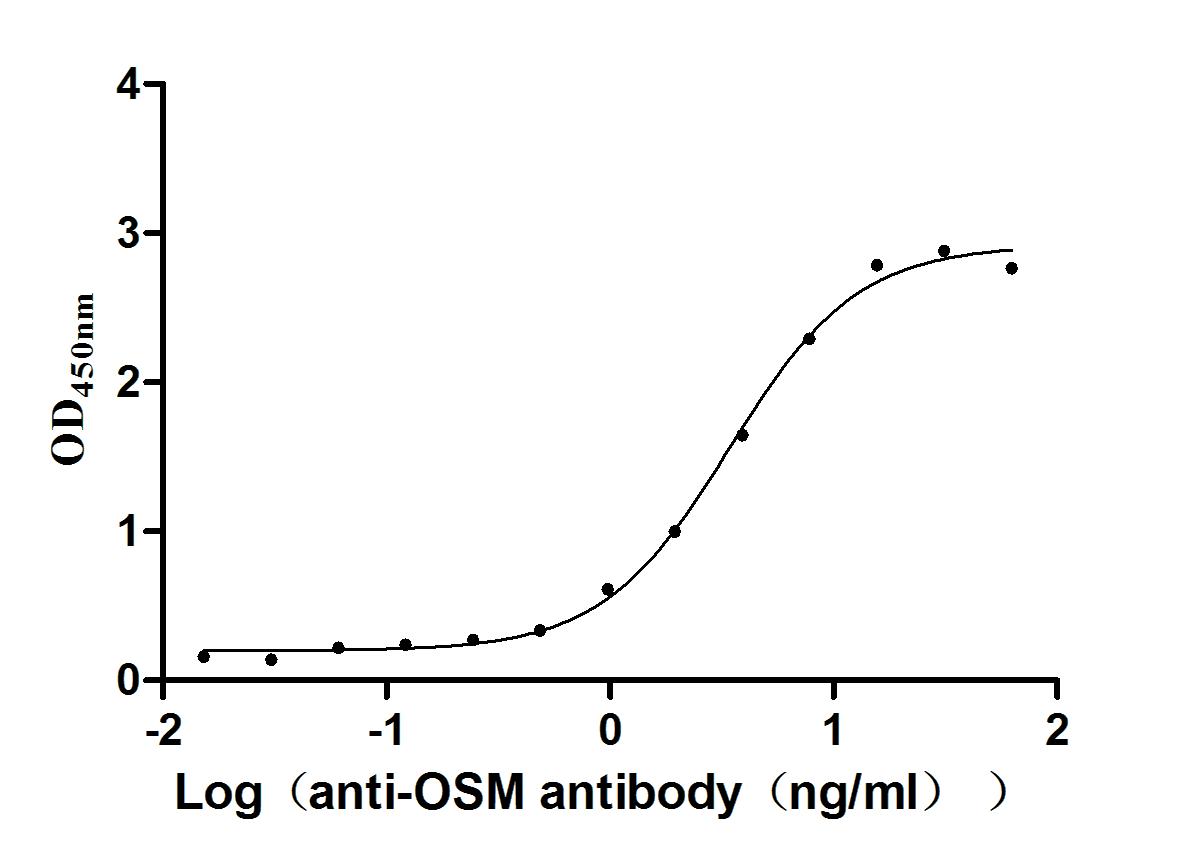
Recombinant Human Oncostatin-M (OSM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
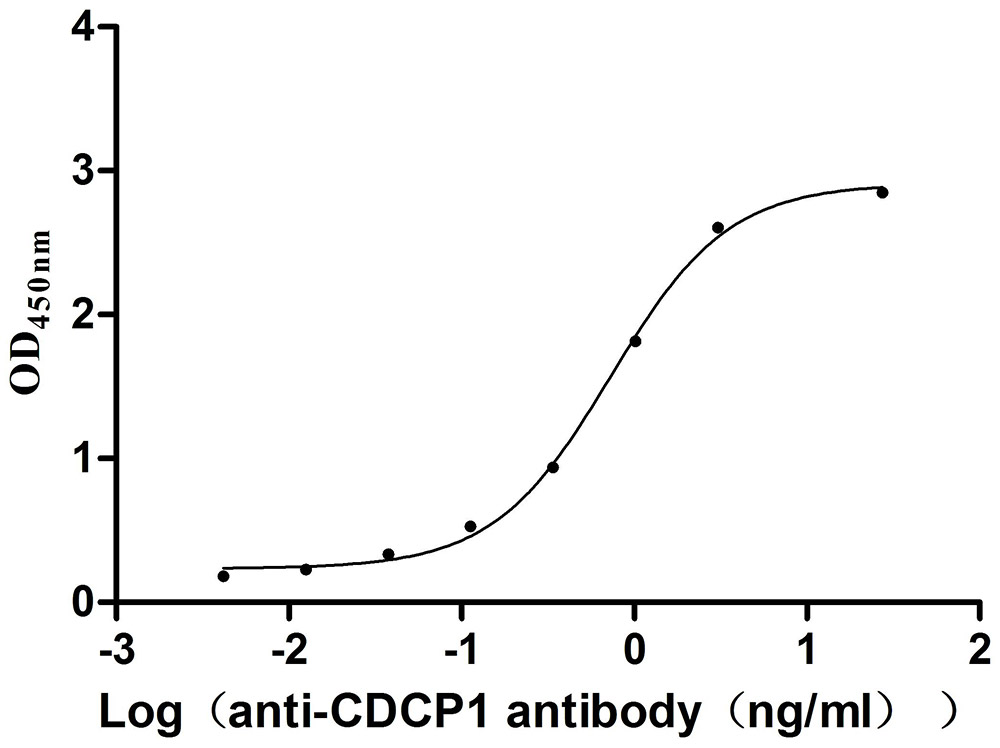
Recombinant Mouse CUB domain-containing protein 1 (Cdcp1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
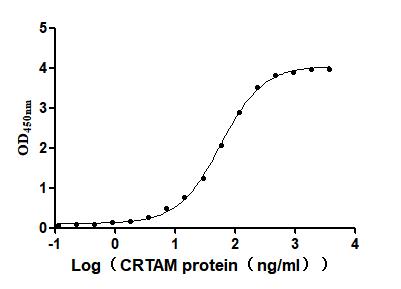
Recombinant Mouse Cytotoxic and regulatory T-cell molecule (Crtam), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
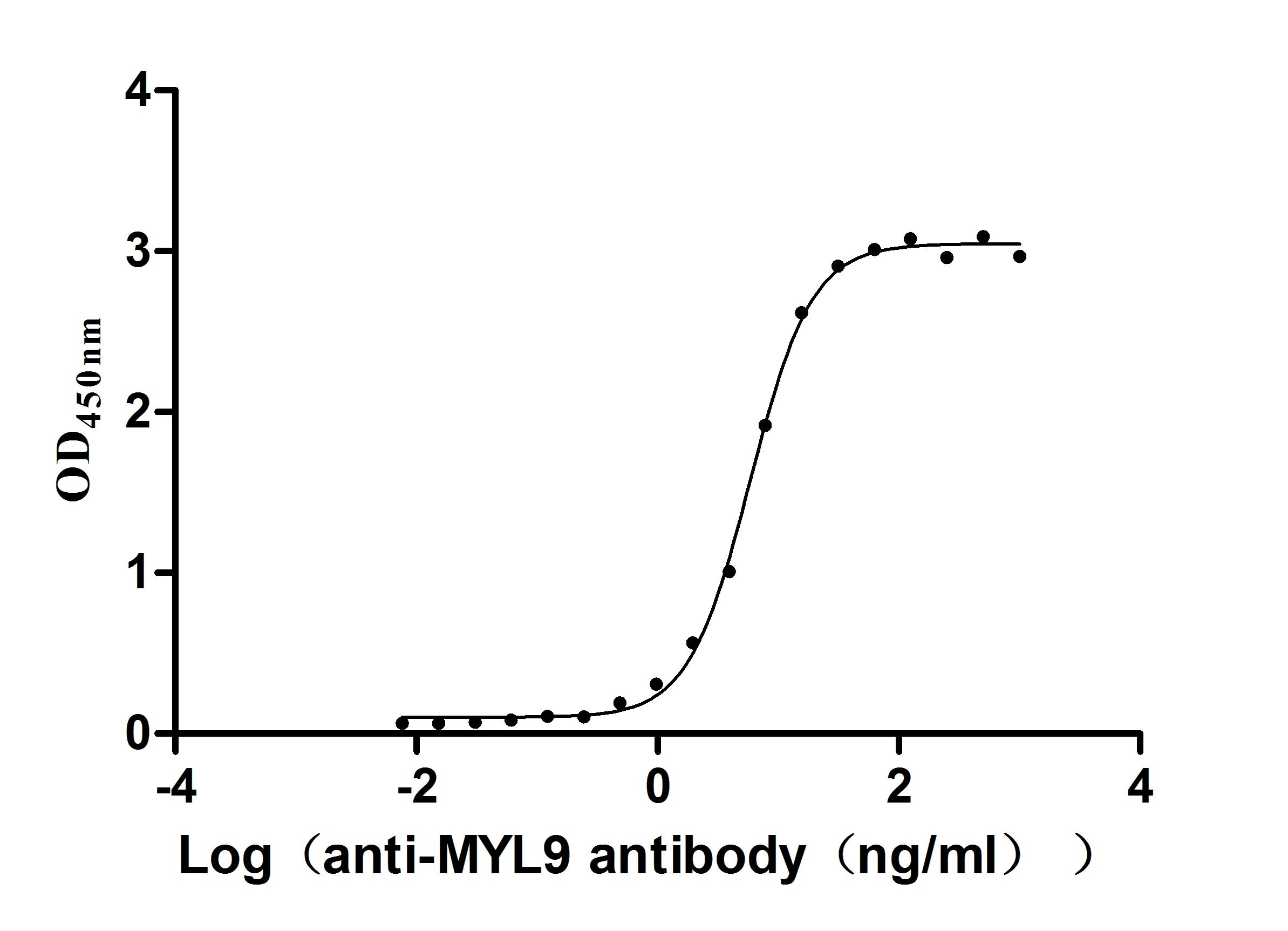
Recombinant Human Myosin regulatory light chain 12A (MYL12A) (Active)
Express system: E.coli
Species: Homo sapiens (Human)
-
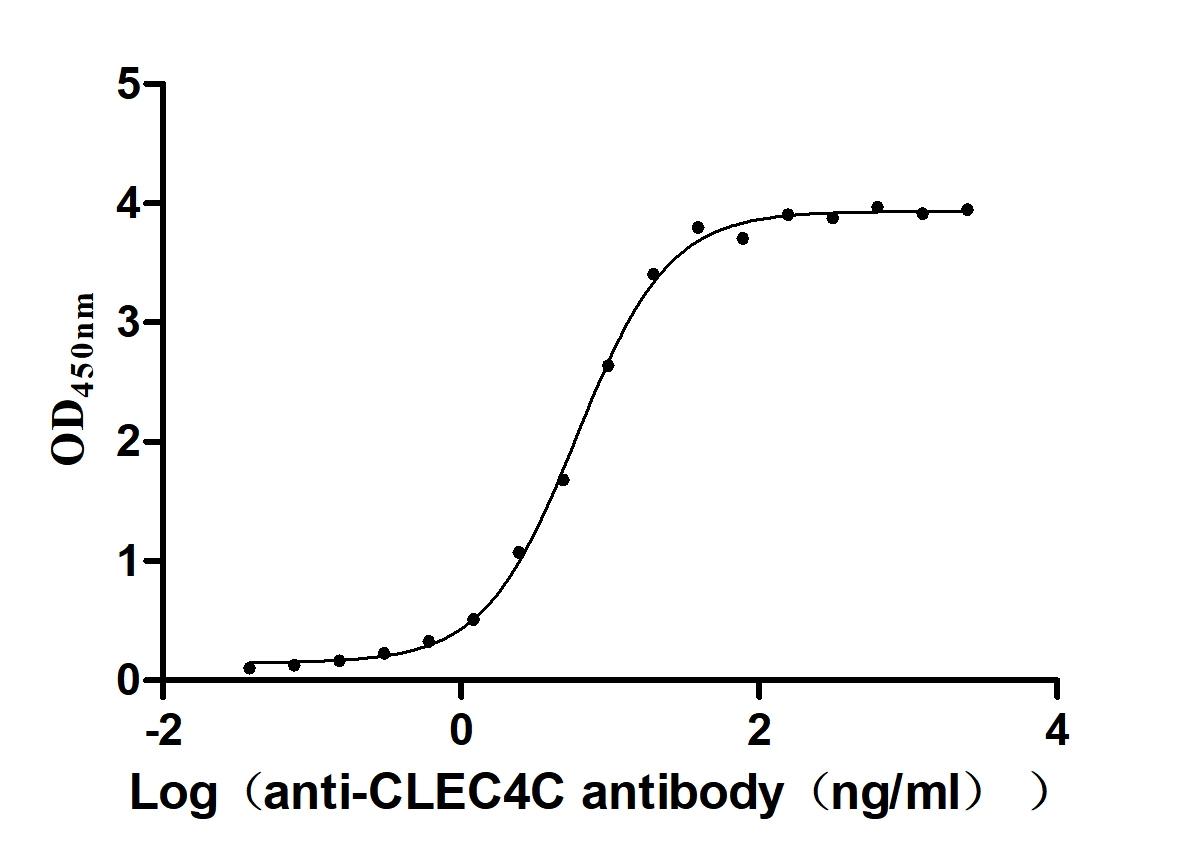
Recombinant Macaca fascicularis C-type lectin domain family 4 member C(CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
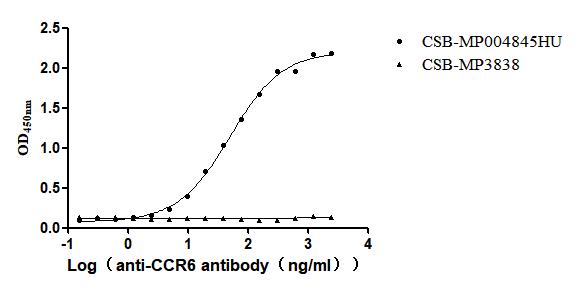
Recombinant Human C-C chemokine receptor type 6(CCR6)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)